Press Release
Aurevia announces strategic split into two independent entities: Aurevia and Labquality
Labquality Days 2026
Welcome to the 50th International Congress on Quality in Laboratory Medicine!
Aurevia
Towards excellence in patient care
As Aurevians, we are the excellence makers: a team of experienced experts in healthcare and health-tech standards and quality. Together, we advance healthcare, pharmaceutical and medical technology development with a future-focused approach, aligned with client needs. Driven by our commitment to care, we contribute quality to the industry and pave the way for safer, more effective patient care worldwide.
Clinical Laboratories
We offer clinical laboratories a wide range of internationally recognised products and services to help them maintain and improve analytical performance.
Pharmaceuticals & Biotech
Our clinical research team provides extensive and high-quality services for pharma and biotech companies.
Medical Devices
Our team of experts serves medical device manufacturers by providing high-quality regulatory and clinical research services for the entire lifecycle of your products.
IVD devices
Clinical research and regulatory affairs services that cover the entire lifecycle of your in vitro diagnostic device.
Latest news

Notice about a change in some of our email addresses
As part of the upcoming company split on 1 January 2026, some of our email domains will change to @labquality.com...

How do you shape regulatory complexity into strategic clarity?
As AI continues to reshape healthcare, clear guidance on health data use is more critical than ever. Building on the...

Fast track for mononational clinical trial applications in Sweden
Starting September 1, 2025, the Swedish Medical Products Agency (Läkemedelsverket) introduced a fast-track process for...

Upcoming webinar: what IVDR Article 5(5) means for your lab
Whether your lab is already working under IVDR and Article 5(5), evaluating tests, or preparing for transition, this...
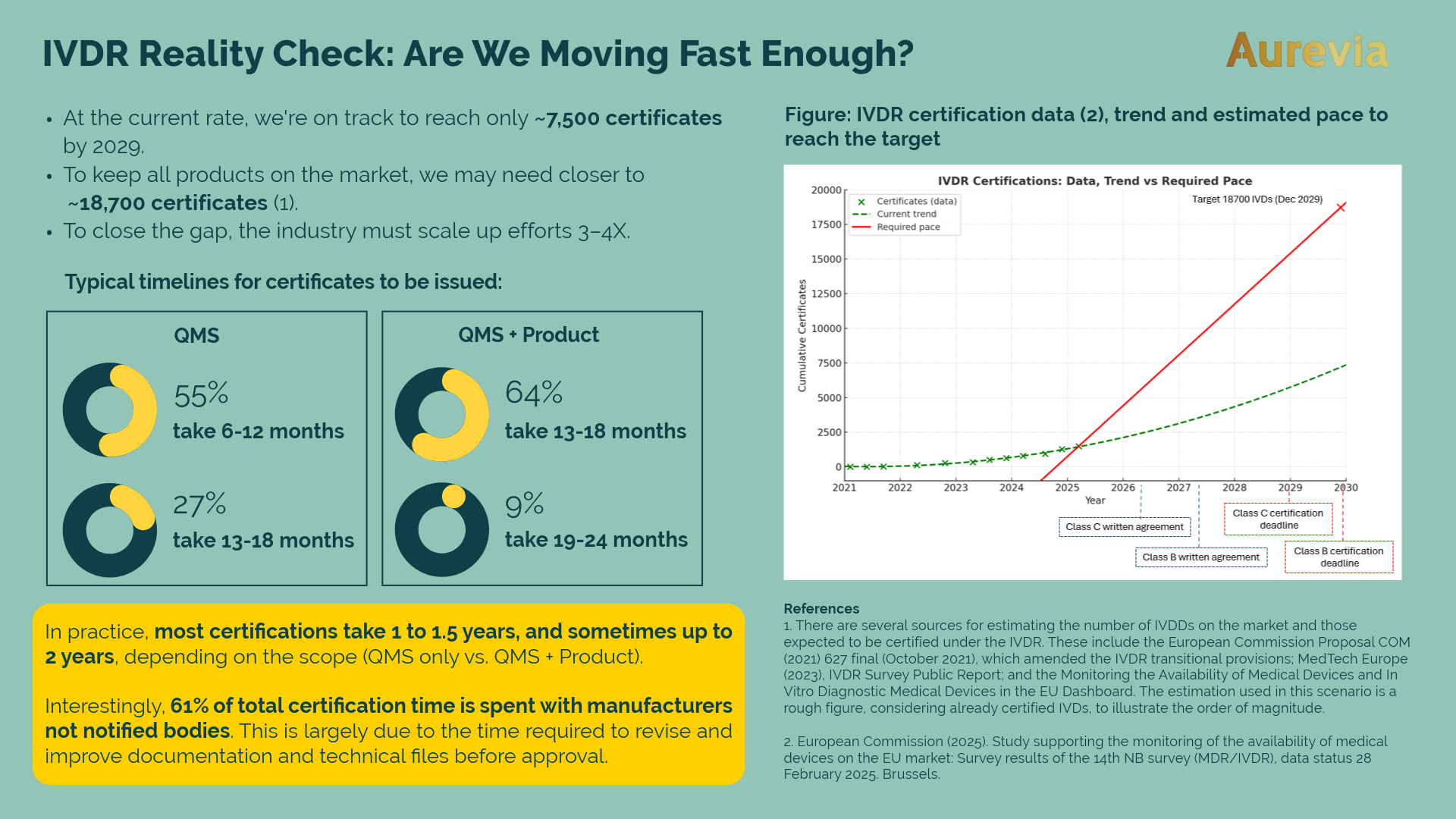
IVDR reality check: are we moving fast enough?
As of February 2025, approximately 1,500 IVDR certificates have been issued. That’s progress — but is it enough? With...