QUALITY ASSURANCE
US FDA QSR
Our expertise with quality systems, including the USA Food and Drug Administration Quality System Regulation, QSR, 21 CFR Title 820 supports your medical device through trials.
Quality System Regulation 21 CFR 820
According to the regulation, it is mandatory for manufacturers to:
- Establish that the quality system is consistent with the complexity of the device, manufacturing processes and size, and the complexity of the manufacturing facility
- Plan to define and implement effective procedures
- Implement what has been documented and is going to be done
- Check the system and make necessary changes (CAPA)
- Act upon changes and ensure they are implemented
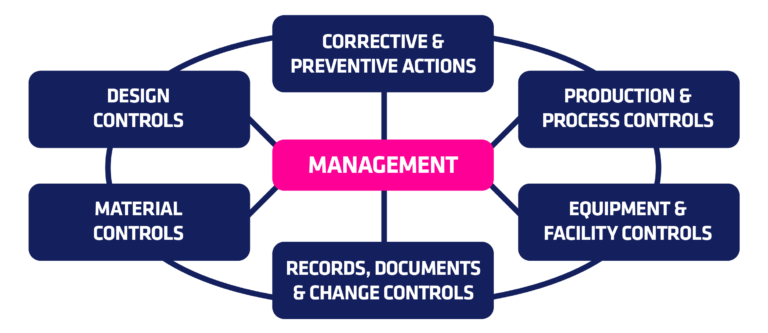
Quality System Regulation 21 CFR 820
According to the regulation, it is mandatory for manufacturers to:
- Establish that the quality system is consistent with the complexity of the device, manufacturing processes and size, and the complexity of the manufacturing facility
- Plan to define and implement effective procedures
- Implement what has been documented and is going to be done
- Check the system and make necessary changes (CAPA)
- Act upon changes and ensure they are implemented
How can we help?
We can assist you with
- Planning the QMS together with the manufacturer according to the QSR regulation
- Identifying all processes needed for their QSR
- Preparing the Quality Manual, QMS process descriptions, templates, databases, and other documentation needed together with the manufacturer
- Choosing and implementing electronic QMS when needed
- Maintaining and continually improving your QMS
- Daily QMS activities including nonconformances, feedback, complaints, internal and supplier audits, vigilance, and many others
- Offering customized and open training for medical device QMS
Explore our services

Quality Management System ISO 13485
Setting up quality management systems (QMS) for medical device manufacturers.

US FDA 21 CFR Title 820 (QSR)
We know quality systems, including the USA Food and Drug Administration Quality System Regulation, QSR, 21 CFR Title 820.

QMS Improvement and Gap Analysis
Improvement of medical device manufacturer’s quality management system (QMS) and GAP analysis.
Latest news
Read the latest news from the world of quality.

QAdvis and Scandinavian CRO join Aurevia to strengthen clinical research and regulatory expertise
Scandinavian CRO (SCRO) and QAdvis will now operate under the name of Aurevia.

Meet us at ESCMID 2025 in Vienna
We will be at the ESCMID 11-15 April 2025 in Vienna, Austria.

Aurevia appoints Jonna Pelanti as the new Head of EQA business area
We are delighted to announce the appointment of the new Head of the EQA business area Jonna Pelanti.


Aurevia appoints Ulrika Hammarström as the new Head of CRO business area
We are delighted to announce the appointment of the new Head of the CRO business area Ulrika Hammarström.
