Access the European Union (EU) – Partner with Aurevia for your EAR representation
Aurevia is an expert in medtech quality system management, regulatory, compliance and more. One of our key competences is also to assist non-EU manufactures of medical devices to comply with European directives/regulations. We have a large international network within the industry and is a member of the European Association of Authorised Representatives (EAAR).
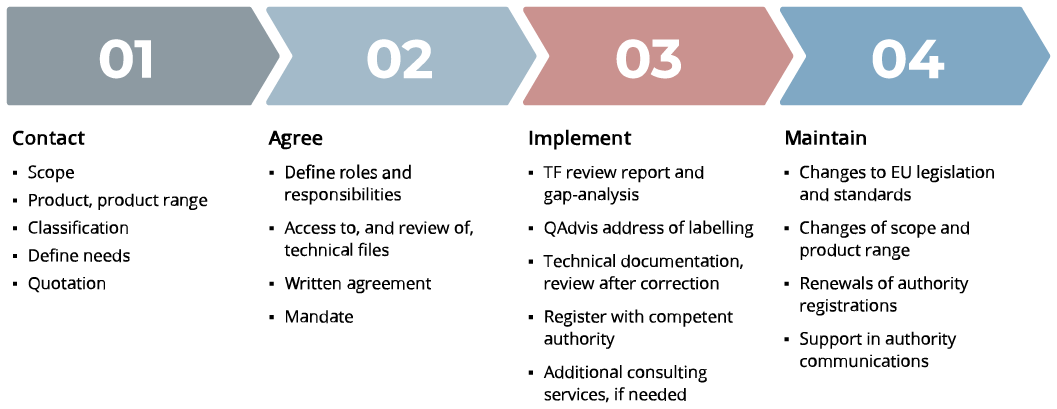
The Process – From Contact to Maintenance
Explore our services

Regulatory plans
Our experienced professionals help medical device manufacturers develop regulatory plans that support their entire product lifecycle.

Technical documentation
All medical devices must have documentation available to support its compliance to applicable regulations. Aurevia supports you in planning and developing your technical documentation.

Electrical Safety IEC 60601
IEC 60601-1 is the basic international safety standard for medical electrical devices and systems.

Biological Safety ISO 10993
The international standard for the biological safety evaluation process and preparing biological evaluation documentation for medical device manufacturers.

IVDR compliance for in-house developed tests
Laboratories developing IVDs in-house must maintain technical documentation and Quality Management Systems to comply with the IVDR and national law.

IVD performance evaluation
In vitro diagnostic medical device (IVD) regulations are set in the EU In Vitro Diagnostics Regulation (IVDR).

Qualification and classification of medical devices
Our team’s versatile experience and the available tools help in bringing clarity to the most challenging cases.

Medical software
Let our experts help you understand and adhere to medical device software regulations throughout the entire lifecycle.

Risk management ISO 14971
We can help you setup and implement a risk management system and prepare risk management documentation.
Latest news

Regulatory strategy vs. regulatory intelligence


QAdvis and Scandinavian CRO join Aurevia to strengthen clinical research and regulatory expertise

Meet us at ESCMID 2025 in Vienna

