Risk Management
We provide set up and implementation of a risk management system and prepare risk management documentation for your products.
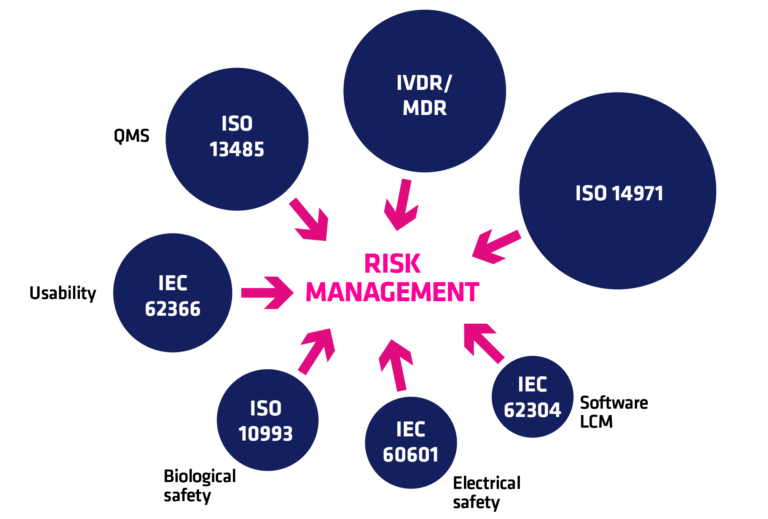
Risk management with medical devices
A risk-based approach is a core element of medical device regulations. Medical device risk management is most conveniently performed following ISO 14971, which is harmonized with Regulations 2017/745 (MDR) and 2017/746 (IVDR) and is US FDA recognised consensus standard.
Performing a risk analysis is a process that produces a risk management file for each medical device. It consists of a risk management plan, risk analyses, and risk management report. It should cover a wide array of different risk categories such as cybersecurity risks and usability risks.
How can we help?
- Writing the risk management process description in accordance with applicable standards and regulations
- Implementing and maintaining the risk management process
- Preparing risk management plans and reports
- Covering all risk analysis aspects (e.g., mfg processes, quality processes, design, usability, software, cybersecurity)
- Determination of benefit-risk
- Answering the risk management related questions raised by notified bodies and authorities
- Offering customized and open training for medical device risk management

Explore our services

Regulatory plans
Our experienced professionals help medical device manufacturers develop regulatory plans that support their entire product lifecycle.

Technical documentation
All medical devices must have documentation available to support its compliance to applicable regulations. Aurevia supports you in planning and developing your technical documentation.

Electrical Safety IEC 60601
IEC 60601-1 is the basic international safety standard for medical electrical devices and systems.

Biological Safety ISO 10993
The international standard for the biological safety evaluation process and preparing biological evaluation documentation for medical device manufacturers.

IVDR compliance for in-house developed tests
Laboratories developing IVDs in-house must maintain technical documentation and Quality Management Systems to comply with the IVDR and national law.

IVD performance evaluation
In vitro diagnostic medical device (IVD) regulations are set in the EU In Vitro Diagnostics Regulation (IVDR).

Qualification and classification of medical devices
Our team’s versatile experience and the available tools help in bringing clarity to the most challenging cases.

Medical software
Let our experts help you understand and adhere to medical device software regulations throughout the entire lifecycle.

Risk management ISO 14971
We can help you setup and implement a risk management system and prepare risk management documentation.
Latest news

QAdvis and Scandinavian CRO join Aurevia to strengthen clinical research and regulatory expertise

Meet us at ESCMID 2025 in Vienna

Aurevia appoints Jonna Pelanti as the new Head of EQA business area


