Solutions for Medical Device Manufacturers
Solutions tailored to your needs
Our quality assurance, regulatory affairs and clinical investigation experts work together to create the most efficient solutions for you: We are a multidisciplinary team with solid industry experience. Our flexible professionals are ready to deliver service, and always work with the customer at the centre. Whether a one-time assignment or a long-term collaboration, we support you in every phase of your regulatory & research journey to reach your business goals.
We provide you with support starting from the first idea of the product until the end of the product life cycle.
QA/RA Services for Medical Device Industry
We help you manage any new guidance, regulations and standardisation. Our highly qualified QA/RA professionals have the experience and expertise to provide you with effective and tailored solutions.
Quality Manager as service
Outsourced Quality Manager service frees up your resources. Your Quality Manager works as part of the organization, collaborating with the leadership and others to ensure regulatory compliance, and manages your QMS.
Project-based QA/RA support
Our consultants support your QA/RA operations together with your QA/RA team. The service can be fully customized, potentially including for example
- regulatory strategies to adapt changes in the regulative environment
- technical documentation
- new product design control support
- global registrations and related documentation updates
Are you expanding to new markets?
Explore our services for the lifecycle of a medical device
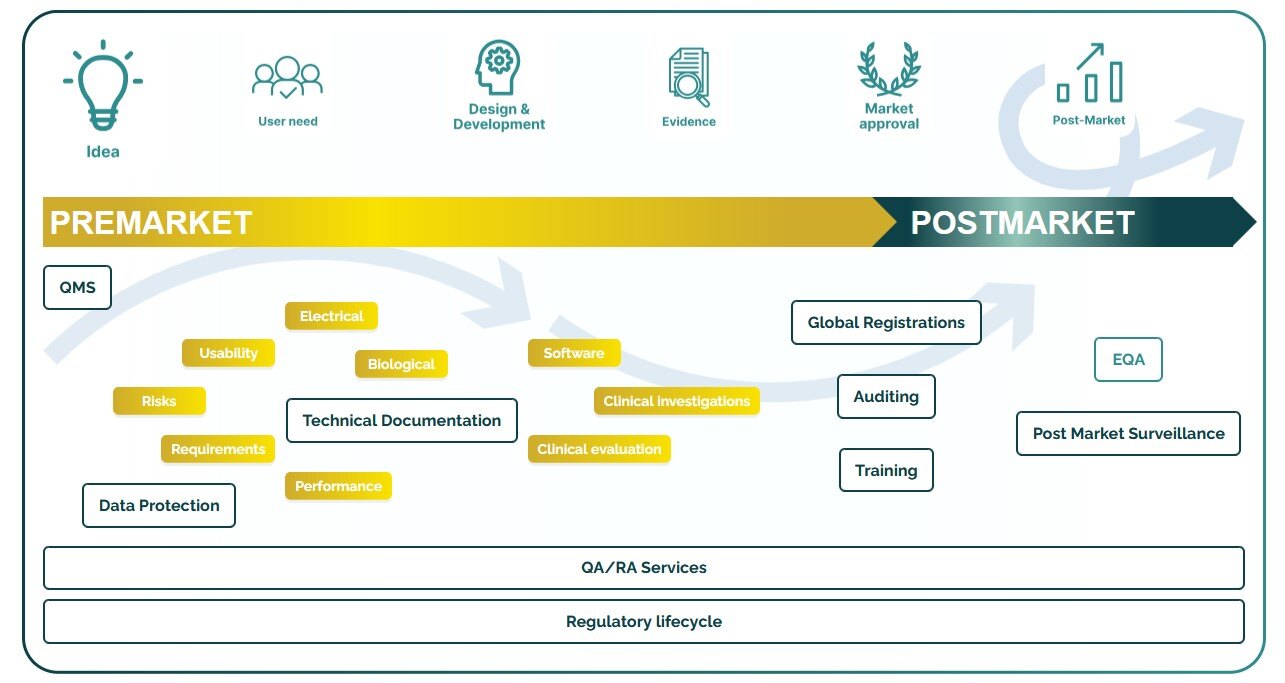
Premarket
Postmarket
Medical Device CRO
We are a full service CRO working not only with medical devices but also IVDDs and pharmaceuticals.

Data Management
We are your one-stop shop for all clinical investigation data management needs.
- General data management
- Data protection
- Biostatistics

Medical writing
We have experience in merging data and regulatory requirements into compliant documentation across the entire lifecycle of the device.
- Regulatory documents for clinical investigations
- Publications

Clinical Evaluation
A clinical evaluation is the link between the technical file and clinical investigation. We provide services in the planning, preparation and conduct of clinical evaluations.

Clinical investigation
Our offering covers planning, development, conduct and reporting of medical device clinical investigations in accordance with the ISO 14155:2020 standard and considering MDR and national requirements.
See also
Our experience with different indications and medical devices. Read more >>
